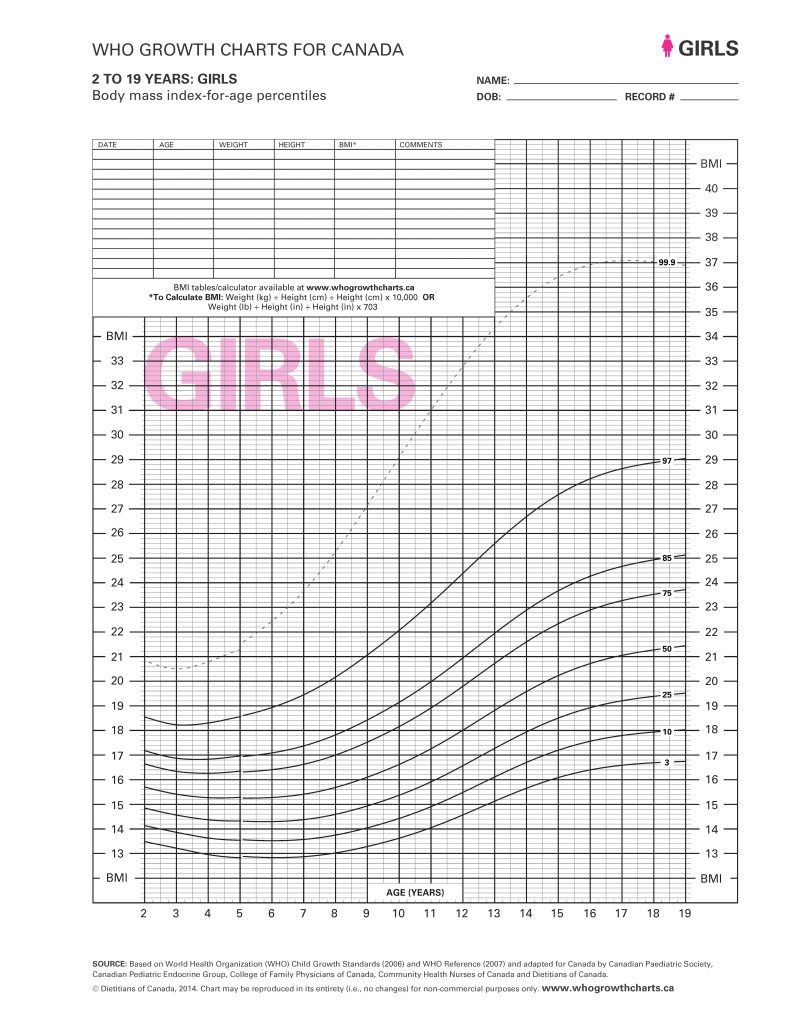
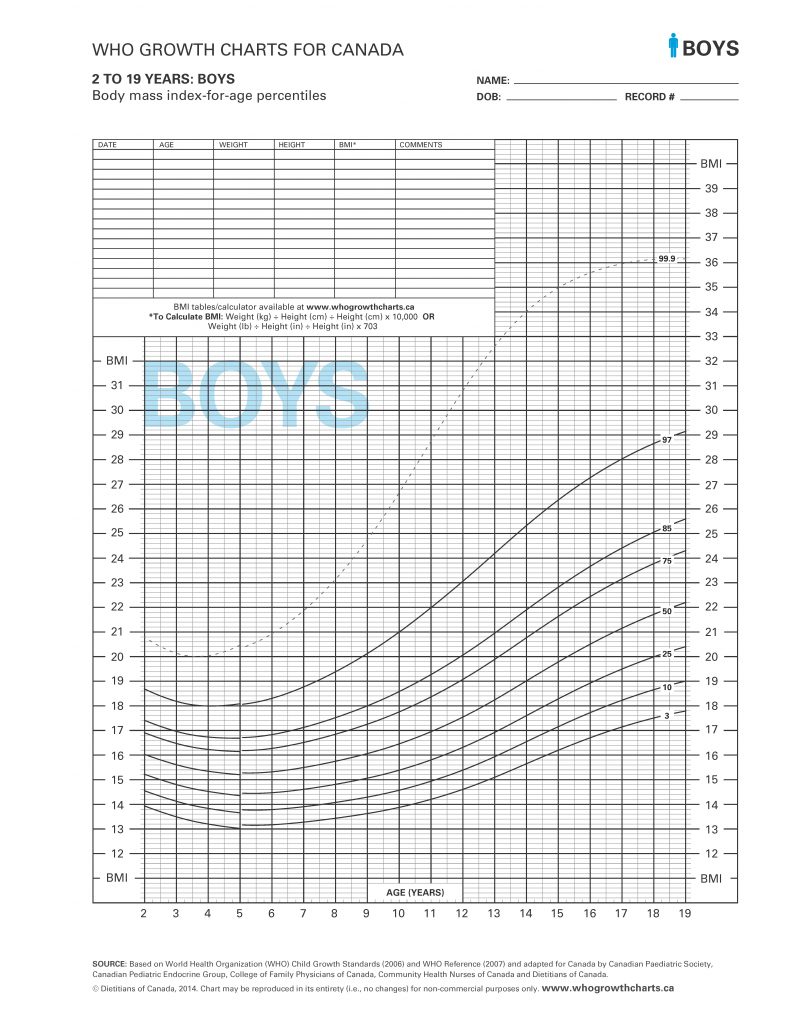
Terms of Use
By accessing and using this website, you agree to be bound by the terms of use, disclaimer and limitation of liability (“terms of use”) set out below.
If you do not agree to be bound by any of these terms of use immediately exit this website and do not access any other pages. These terms of use may change from time to time without notice and it is your responsibility to check for updates. By using this website you are agreeing to be bound by the then current version of these terms of use. The last revision date for these terms of use is set forth below.
Information
Information on this website is provided for general educational information purposes only. It does not constitute medical advice and is not intended to be a substitute for advice given by your physician or other qualified health care professional. Always seek the advice of your physician or other qualified health care professional with any questions you may have. Never disregard medical advice or delay seeking medical advice because of something on this website. Information contained in this website or any websites to which a link is provided should be used only under the supervision of an appropriately qualified physician. Please consult with your physician before making any decision regarding treatment and/or medication. If you think you may have a medical emergency call your doctor or 911 immediately.
Reliance on any information on this website is solely at your risk.
Disclaimer and limitation of liability
Any information created by us is believed to be reliable when posted. However, we do not guarantee or warrant the quality, accuracy, completeness, timeliness, appropriateness or suitability of the information provided. Reliance on any information appearing on the website is at your own risk. We expressly disclaim all warranties, representations and conditions (express or implied) regarding use of the website or the information on this website. We assume no obligation to update the information or advise on further developments concerning topics mentioned. The information is supplied “as is”, “as available” and may contain errors. The information may be changed from time to time without notice. Certain portions of the information may have been contributed by other persons and should not be assumed to have been reviewed or endorsed by us. The mention of specific products or services on this website does not constitute or imply a recommendation or endorsement by us, unless explicitly stated.
The BC Children’s Hospital, Provincial Health Services Authority and content providers including without limitation the health care professionals who contribute to the information on this website shall have no liability whether direct, indirect, consequential, contingent, special or incidental related to or arising out of or in connection with the use of this website or your reliance on the information, whether in an action of contract, negligence or other tortious action. Anyone using this information does so at his or her own risk, and by using such information agrees to indemnify the BC Children’s Hospital, Provincial Health Services Authority, and content providers from any and all liability, loss, injury, damages, costs and expenses (including legal fees and expenses) arising from such person’s use of this website, information on this website, and any information on websites linked from this website.
Links
Any links we provide to other websites are provided as a reference to help you identify and locate other internet resources that may be of interest. These other websites were independently developed by other parties and we do not assume responsibility for the accuracy or appropriateness of the information contained, or endorse the viewpoints expressed, at such other websites. Your use of any third party website is at your own risk and is subject to the terms of use of such website.
Use of this website
You accept that this website and the information available on it may be unavailable from time to time due to routine maintenance, upgrades, hardware or software malfunctions, repairs, outages or other unforeseen circumstances beyond the reasonable control of BC Children’s Hospital or Provincial Health Services Authority.
You are prohibited from posting malicious or unauthorized code (e.g., viruses, bots, worms, spyware, Trojan horses, etc.) or other potentially harmful material to this website that may interrupt, damage or limit the functionality of this website or the use of this website by others. You are prohibited from using this website for spamming, phishing or other similar schemes.
Intellectual property
Provincial Health Services Authority owns or has authority to use all information and content on this website, including any icons, images, text, illustrations, computer software and code. You are granted a limited licence to use this website for your personal, non-commercial use only, provided the information on this website is not modified and all copyright and other proprietary notices are retained. Any other use is strictly prohibited without permission from Provincial Health Services Authority. None of the information on this website, in whole or in part, may be reproduced, republished or re-disseminated in any manner or form without prior written consent from Provincial Health Services Authority. Nothing contained in these Terms of Use shall be construed as conferring copyright in any information provided on this website.
Any names, words, titles, phrases, logos, designs, graphics or icons appearing on this website may be registered or unregistered trade-marks, official marks, service marks or trade-names and may be the property of Provincial Health Services Authority or third parties. Though third party marks may be used by Provincial Health Services Authority subject to a licence agreement, the display of a third-party’s trade-marks does not indicate or imply any relationship between Provincial Health Services Authority or BC Children’s Hospital and that third party, or indicate that Provincial Health Services Authority or BC Children’s Hospital approve of any of the products or services used in association with that trade-mark. Nothing contained herein implies that a licence has been granted to you in respect of any official marks, trade-marks, service marks or trade names displayed on this website.
Entire Agreement
If any part of these Terms of Use is found void and unenforceable, it will not affect the validity of the balance of the remaining Terms of Use, which will remain valid and enforceable. These Terms of Use set forth the entire understanding and agreement between Provincial Health Services Authority and BC Children’s Hospital and you with respect to your use of this website and the information contained on this website.
Choice of law
The laws in effect in the Province of British Columbia shall govern these terms of use and any dispute or claim based on your use of the website. You agree that any action or proceeding relating to this agreement or your use of the website shall only be brought by you in the courts of the Province of British Columbia. The website is intended for use by residents of British Columbia, Canada only.
These terms of use set forth the entire understanding and agreement between us and you with respect to your use of the website.
Copyright © 2020 Provincial Health Services Authority. All Rights Reserved.